Phagenyx® for Healthcare Providers

Dysphagia is Common, Costly, and Harmful
Dysphagia is a frequent consequence of stroke and is associated with longer hospital stays, increased costs and aspiration-related complications. Conventional treatments may not target the disruption to the sensory nerves or brain areas that control swallowing, limiting the potential for functional recovery.
45%
of stroke patients suffer from persistent dysphagia1
10+ day longer
average length of stay2
8x higher mortality
at 3 months3
Severe dysphagia can cause:
- Excessive secretion pooling
- Significant pharyngeal residue
- Impaired airway protection including silent aspiration
Which increases risk of:
- Intubation
- Inability to decannulate
- PEG tube
Current Dysphagia Treatment Limitations
Current Treatments
Compensatory Interventions
- No food by mouth (NPO) or modified diets
- Alternate means of nutrition, including PEGs
- Pneumonia prevention strategies
Rehabilitative Interventions
- Swallowing exercises and maneuvers
- Respiratory muscle strength training
Current Treatment Limitations
Targets Symptoms, Not Root Cause
No direct evidence of neuroplasticity
Limited Evidence-Base
Mixed evidence on current standard of care. Lack validated treatment protocols
Requires Active Patient Engagement
Makes early intervention more challenging
The Phagenyx System
Introducing the first and only clinically validated neuromodulation system using pharyngeal electrical stimulation (PES) to retrain the brain and restore swallowing control.
Faster Return to Oral Nutrition
6-day faster return to oral nutrition and 50% fewer patients dependent on feeding tubes at discharge4
Reduced Aspiration Risk
Greater than 2x improvement in swallowing safety5
Shortened Length of Stay
8 fewer days in the hospital4 and 4x more patients ready for early decannulation6
Phagenyx Patient Selection
Patients with any of the following characteristics of severe dysphagia are candidates for Phagenyx treatment.
Abnormal laryngeal/pharyngeal sensation
or
Impaired swallow safety and efficiency
or
Reduced or absent spontaneous swallowing
Active patient engagement and participation is not required for Phagenyx treatment
What is Pharyngeal Electrical Stimulation (PES)?
A new approach to improving swallow safety & efficiency
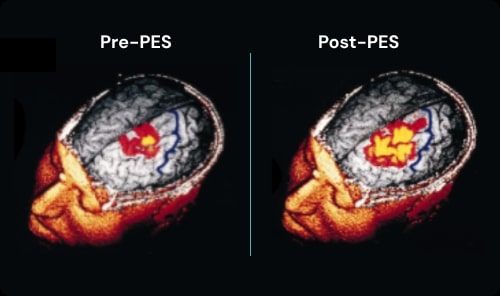
Pharyngeal Electrical Stimulation (PES) is a neuromodulation therapy with demonstrated efficacy in neurogenic dysphagia. By activating pharyngeal afferent pathways, PES engages central swallowing networks, facilitating reorganization and promoting earlier, safer functional recovery.
- Treats the neurological causeAddresses swallowing difficulty at its source by stimulating pharyngeal sensory nerves to reactivate brain pathways critical to swallowing.
- Promotes neuroplastic recoveryTriggers afferent signaling that facilitates cortical reorganization and supports the restoration of functional swallowing control.
- Grounded in scientific rigorFoundational research and peer-reviewed publications prove brief pharyngeal stimulation induces lasting neuroplastic changes in cortical activity associated with swallowing control.
- Validated with clinical evidenceRandomized controlled trials demonstrate improvement in swallowing safety and efficiency, resulting in faster return to oral nutrition, reduced aspiration risk and shortened length of stay.
The Science of PES
Patient Story
Hear from Michael, a Certified Master Baker, about how Phagenyx therapy helped him regain the ability to swallow food after a massive stroke.
6 days
Cost Effectiveness of Phagenyx Treatment
Phagenyx treatment for patients with severe dysphagia may result in annual cost savings.
Fewer Reintubations
Fewer patients required reintubation after Phagenyx treatment, compared to control7
Reduction in ICU Length of Stay
Patients treated with Phagenyx required 5.4 fewer days in the ICU, compared to control7
Fewer Days in the Hospital
Patients treated with Phagenyx were discharged from the hospital 8 days faster, compared to control7
Service & Support
With you every step of the way
Program Initiation
Align stakeholders, confirm project timeline, assess site readiness and coordinate logistics to ensure a smooth and successful Phagenyx launch.
Training and Education
On-site comprehensive training for healthcare professionals, including guidance on patient selection, to support successful Phagenyx use.
Clinical Implementation
Onsite and virtual support, tools and best practices, and outcome tracking to optimize Phagenyx success.
Program Optimization
Supports long-term success through protocol integration, ongoing support, outcome reviews, and multiservice integration.

Bring Phagenyx to Your Hospital
Ready to transform your approach to post-stroke dysphagia recovery? Let’s start the conversation.
Questions?

Treatment Questions
What type of patient is a good candidate for Phagenyx treatment?
Phagenyx may be appropriate for patients with reduced or absent spontaneous swallowing, impaired swallowing safety or efficiency, or abnormal sensation in the pharynx or larynx.
Can Phagenyx be used for patients with severe dysphagia who have not had a stroke?
In the US, the Phagenyx is indicated for use in patients with severe dysphagia post stroke.
Can Phagenyx be used for patients with a loop recorder or pacemaker?
No, loop recorders and pacemakers have active electrical components and are contraindicated for Phagenyx treatment.
Availability Questions
I have a patient whom I think would benefit from PES therapy. Where can I refer them?
In the US, Phagenyx is only available in acute care, inpatient settings. Please contact us at [email protected] for additional details.
How can I get this in my hospital?
To speak with a Phagenyx representative, contact us at [email protected].
How can I get this at my rehab center?
Currently, Phagenyx treatment is only being offered in hospital in-patient settings.