Phagenyx® for patients & families
Phagenyx is a non-surgical therapy that helps restore safe, effective swallowing and supports faster recovery for patients with severe dysphagia after a stroke

A New Way to Support Swallowing Recovery

What is dysphagia?
Dysphagia is the medical term for difficulty swallowing, which is common after stroke, brain injury, or serious illness. It can lead to longer hospital stays and serious health risks like food or liquid entering the airway. Traditional treatments may not fully treat the brain pathways involved in swallowing, which can slow down recovery.
What is PES?
Pharyngeal electrical stimulation (PES) is a targeted therapy that delivers small electrical pulses to the swallowing nerves in the throat to re-establish the communication between these nerves and the brain.
What is Phagenyx?
Phagenyx is the first treatment of its kind to use pharyngeal electrical stimulation (PES) to help retrain the brain and restore safe, effective swallowing. This non-surgical therapy is delivered at the bedside in just a few minutes a day and is designed to support a faster, safer recovery for people with severe swallowing difficulty.
How Phagenyx Works
Phagenyx uses pharyngeal electrical stimulation (PES) to retrain the brain and restore swallowing control.
Stimulation of swallowing nerves
Swallowing control is restored
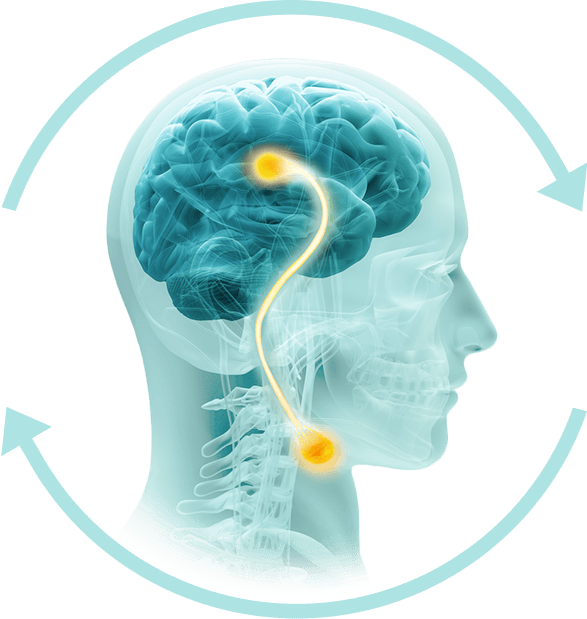
Signals transmitted to the brain
Brain relearns how to swallow
Swallowing control is restored
Stroke Recovery Starts Here
Phagenyx helps restore safe swallowing and support faster recovery.
Additional Resources
Questions?

Treatment Questions
How can I get Phagenyx treatment?
Phagenyx treatment is only available to hospital in-patients with severe dysphagia following a stroke. It is not currently available on an outpatient basis. Treatment is delivered by a trained clinician and is not approved for home use.
What does it feel like during treatment?
Patients may feel a tingling sensation in their throat during treatment. Stimulation levels are tested prior to treatment to ensure it is strong but tolerable.
Is surgery required for Phagenyx treatment?
No, Phagenyx treatment does not require surgery.
Availability Questions
My loved one has dysphagia caused by cancer…would Phagenyx help them?
Phagenyx is indicated for the treatment of severe dysphagia following stroke. Its use in patients post-cancer treatment is considered off-label. We do not have any published data in this patient population.
My loved one has dysphagia caused by Parkinson's…would Phagenyx help them?
Phagenyx is indicated for the treatment of severe dysphagia following stroke. Treatment of patients with Parkinson’s disease is considered off-label. We do not have any published data in this patient population.
Would Phagenyx be suitable for my child, who is 15 years old?
Phagenyx is indicated for adult patients only. We do not have safety or efficacy data in pediatric patients (18 years old or younger), so use of Phagenyx would be considered off-label.





